The GLOBIZ – Global Virtual Healthcare Expo got great traction owing to webinar organized by Indian Pharma Machine Manufacturers’ Association (IPMMA) in association with FICCI. The webinar on “Pharmaceutical Technology – The Way forward” was sponsored by Glatt Engineering Pvt. Ltd. It was well received by the viewers and Expo participants. Harshit Shah, Secretary IPMMA warmly welcomed the participants and set the tone right for the insightful webinar. The eminent speakers Anuvrat Singhal, MD Glatt Engineering Pvt. Ltd. and Dr. Mahesh Balghat, COO – Syngene International Ltd shared very insightful presentation depicting a picture of the future of Pharma technology.
Kaushik Desai Advisor, IPMMA and Moderator of the webinar initiated the conversation on the buzzword Pharma 4.0 with new dosage forms and new drug delivery systems, and the market drivers of technology in competitive global markets.
Anuvrat Singal, MD – Glatt Engineering Pvt. Ltd. shared that through continuous innovation Glatt was able to diversify into multiple segments with a workforce of 3000+ driven members spread across 14 countries worldwide. When we dive deep into projects, we need to identify the product therapeutic product categories to be manufactured.
In today’s pandemic situation, automation and Industry 4.0 are new catchwords which needs to be factored in early stages of projects. This not only provides ease of operation but also opens possibility to expand in a logical manner.
Mr Singal shared case study of a Pharma Future ready facility deploying Automatic Storage and Retrieval Solution (ASRS). The facility had to be pharma future ready deploying automation aspects and other key internet of things. A major challenge faced was handling nearly 10 to 12 tons of product every day, and the equipment technology which was also quite critical to the design of the mega facility. We looked at the main equipment, the fluid bed dryer which went as high as 25 meters.
This was the heart of the entire plant. In other words, the other process units were built around it. We opted for vertical model, starting at one level and descending down the levels in order to utilize gravity to our advantage. The Automatic Storage and Retrieval Solution (ASRS) solution was deployed because the volumes were beyond the capacity of conventional warehouse technology. Recently ASRS is penetrating the pharma industry which is more pertinent in the e-commerce and the auto manufacturing industry.
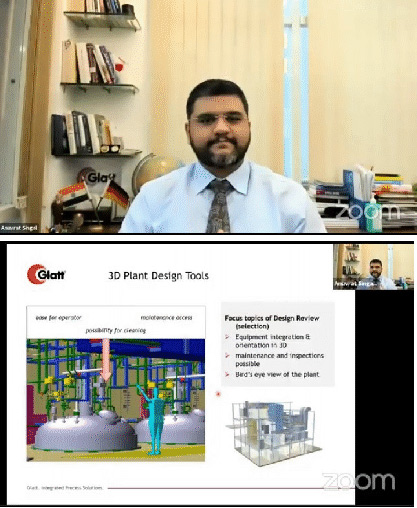
In other case study, Mr. Singal dwelled upon the project entailing 250+ different types of product to be manufactured per annum with a three-shift model. The client was targeting 14 plus billion tablets and capsules. Considering the volume of products, the vertical model was not the right choice. Hence horizontal approach was adopted in which the products pass in a very linear manner within this facility. Another major challenge encountered was the transferring of dirty utensils from various pockets of the plant to a centralized place for washing and then back. Along with the centralized washing, centralized weighing and dispensing solution with the state-of-the-art online automated station gave birth to what is called Automatic Storage and Retrieval Solution (ASRS).
He further added, 3D technology is here to stay for a long time. It enables us to think about the project for ease of access in terms of maintenance, equipment and the material movement while giving us a bird’s eye view in transferring everything from 2D to 3D drawings.
Now Virtual Reality and Augmented Reality are also penetrating the facility where the equipment manufacturer based either in Europe or in the US is able to service the machine in India. We do believe that robots are here to stay because they are fast, they can repeat the same task effectively and supports data integrity. Other than Pharma 4.0,Workforce 4.0 will also be important where it would entail training of operators at shop floor in order to be fully compliant.
The second esteemed speaker Dr. Mahesh Balghat,
COO – Syngene International Ltd shared his views on types of vaccines, how they differ technologically and most importantly what’s India doing in this perspective. He also explained about some of the delivery challenges associated with new technology in vaccines further giving an overview of vaccine manufacturing.
Low cost of manufacturing vaccines is the key driver as it’ll be reaching across all parts of the world. India is the base for doing R&D at a reasonable cost which makes it a great opportunity for India that needs to be leveraged. India also offers a congenial environment for conducting clinical trials through the availability of both subjects as well as scientists throughout the entire development cycle.
Dr. Balghat presented an overview on the regulatory steps involved for a vaccine or any other drug approval. The phenomenal work for COVID-19 vaccine is undercurrent as it is reaching to the tune of thousands of patients that are being subjected to the phase three trials. However, this time it is on a much more accelerated time frame than a typical time frame, subject to regulatory review, emergency use authorization and much more.
He also shared his views on the Single use versus Conventional facility. Elaborating on it, he mentioned that in case of Single use facility OPEX is higher considering consumable like plastic bags and all supporting consumables for the bioreactors. This is the first time ever in India we are doing this kind of vaccine manufacturing for its own population of 1.3 billion. Seeing its urgency, everybody needs to be on a common platform and look at what is possible to bring more and more vaccine manufacturing capacity with reliable supplies which typically takes 10 to 12 years to reach market. Another aspect needs attention is cold supply chain, making it economical and feasible for the vaccine to reach immunization centres.
The highly knowledge sharing webinar concluded with a vote of thanks by Oswin D’Souza, IPMMA committee member and FICCI representative for MSME committee. Mr. D’Souza on behalf of the IPMMA, thanked both the eminent speakers for their excellent insights and also catering to the pertinent questions. He further thanked speakers for sharing about the future of projects and the process of research and technological development for the much-awaited COVID-19 vaccine. He also thanked FICCI for providing this one-of-its-kind Global Virtual Healthcare Expo as a platform for interactive webinar with all the concerned stakeholders from the Indian Pharma Industry. He signed off by thanking all participants for making the webinar a success with their active participation.


Leave a Reply
You must be logged in to post a comment.